Episode 27- “More Squeeze, Please:” Part 2: Clots and COVID
Episode Summary:
In Part 2 of this “Mini Grand Rounds” series, we discuss important side effects of ATII including thrombosis, especially in the setting of COVID.
Show Notes:
Key Points:
“‘More Squeeze, Please’: Part 2: Clots and COVID”:
– In the ATHOS-3 trial, the rates of thrombosis were higher in the ATII group than the placebo group (13% vs 5%; P = 0.015). In addition, more patients in the ATII group than the placebo group were on antithrombotics medications (87% vs 72%; P <0.001)
– A potential mechanism of this higher rate of thrombosis involves the ACE1 and ACE2 angiotensin pathways. When ACE1 cleaves angiotensin I (AT1) to ATII, ATII binds the angiotensin II type 1 receptor, which causes vasoconstriction, inflammation, and thrombosis. However, ACE2 cleaves ATII into angiotensin (1-7), which is a vasodilator and has anti-inflammatory and anti-thrombotic properties (see image below)
– Though ACE2 may sound beneficial in its properties, it also acts as an entry point for SARS-CoV-2 into cells. The binding of SARS-CoV-2 to ACE2 means there is less ACE2 available, leading to higher amounts of ATII and lower amounts of angiotensin (1-7)- a double whammy
– Experts recommend continuing ACE-I and ARB therapy in patients with COVID, as these agents have been shown to reduce thrombosis rates, likely due to their effects on reducing the amount of ATII and up-regulating ACE2
– This creates a paradox: is more ACE2 beneficial as it leads to less ATII and more angiotensin (1-7), or is it harmful due its role as an entry-point for SARS-CoV-2?
– If ATII is so harmful, why are we actively infusing it into any patient, especially someone with COVID? To complicate things, there are case series and case reports that show that ATII has been effective for patients in septic shock with COVID, potentially due to its effect on down-regulating ACE2
– Confused? So are the experts. We will await further studies to elucidate the true effect of the angiotensin pathway on thrombosis rates and efficacy in the setting of COVID
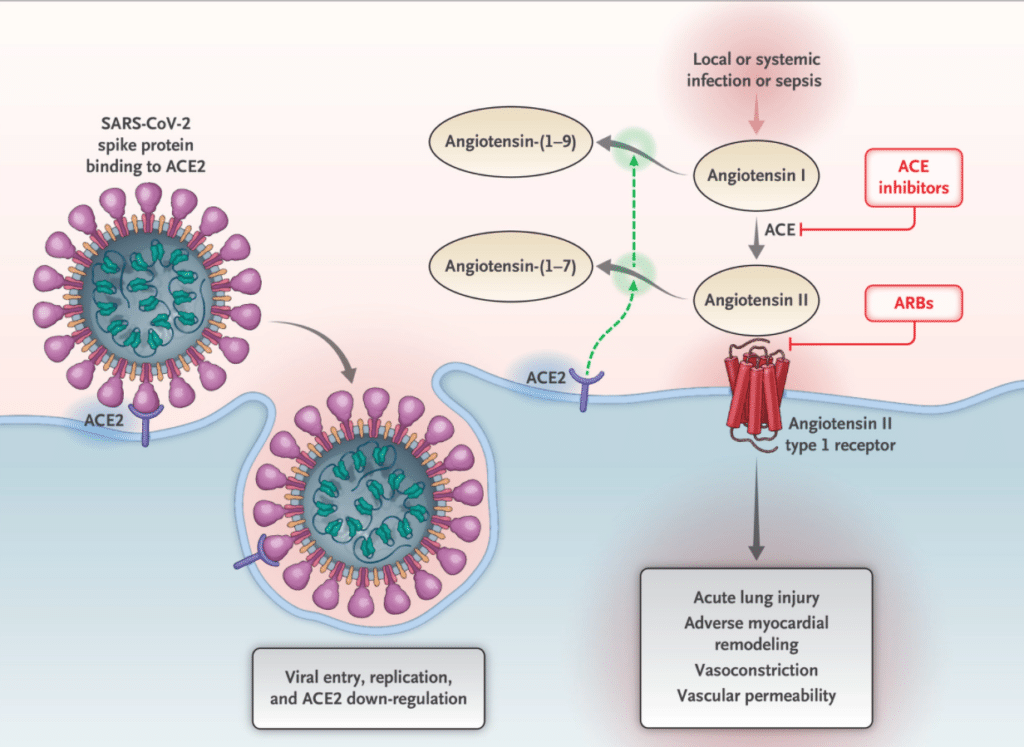
Angiotensin I (ATI) can go down two pathways. One pathway, through the conversion of ATI to ATII by ACE1 ultimately causes vasoconstriction and thrombosis by ATII’s effects on the ATII type 1 receptor. A second pathway, through ACE2, converts ATII into angiotensin 1-7, which is an anti-inflammatory and anti-thrombotic through its effects on the MAS receptor. Unfortunately, ACE2 is also an entry point for SARS-CoV-2 into cells.
Source: Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. NEJM. 2020; 382: 1653-1659
References:
Celi A, Cianchetti S, Dell’Omo G, Pedrinelli R. Angiotensin II, tissue factor and the thrombotic paradox of hypertension. Expert Review Cardiovasc Therapy. 2010. 8 (12)
Transcript:
Hello and welcome to Episode 27 of ER-Rx. Last week, in Part 1 of our “AT II Mini Grand Rounds series,” we reviewed the findings of the ATHOS-3 trial, as well as my own personal experiences with using the agent. In Part 2 of our series, we talk about potential side effects of ATII as well as some important implications of ATII use in the setting of COVID.
In ATHOS-3, adverse drug events of any grade (87% vs 92%) and serious adverse events (61% vs 67%) were similar between the groups, and study infusion was discontinued at similar rates and for similar reasons; including multiorgan system failure and cardiac arrest.
But there is some controversy about these adverse drug events, particularly when we look at rates of thrombosis. Per the published ATHOS-3 trial, only 3 patients (1.8%) in the ATII group and 0 patients in the placebo group developed a DVT. Although supplementary materials didn’t discuss this any further, other reporting states that the rate of arterial and venous thrombosis was actually 13% in the ATII group vs 5% in the placebo group (P = 0.015). And although the authors of ATHOS-3 didn’t mention rates of patients on DVT prophylaxis, data from the manufacturer shows that more patients in the ATII group (87%) than the placebo group (72%, P <0.001) were on antithrombotic medications, which makes the higher risk of thrombosis even a bigger concern. That’s why the FDA advises DVT prophylaxis along with ATII use in your patients.
Is this increased thrombosis rate just due chance, or do we actually have a plausible mechanism here? Well, the exact reason for this potentially higher rate of thrombosis is unclear and deserves further study, we do know from clinical trials that ATII can be a mediator of thrombosis. This is evidenced by data showing that hypertensive patients treated with an ACE-I or an ARB have lower rates of thrombosis- possibly due to less ATII floating around their bodies. We also have cool studies in mice where they showed that mice given ATII infusions had accelerated rates of thrombosis.
But how does this happen? I’ll post an image of the angiotensin pathway onto the errxpodcast Instagram page as well as errxpodcast.com, but I’ll try to briefly explain the mechanism here.
When angiotensin converting enzyme-1 (ACE1) cleaves angiotensin I (AT1) to ATII, ATII binds the angiotensin II type 1 receptor (AT1-R) which causes as we know from ATHOS-3, vasoconstriction and an increase in BP and MAP, but it also causes fibrosis, hypertrophy, and inflammation. Think of this as the “bad pathway” in the setting of thrombosis.
However, a separate enzyme, one that maybe wasn’t mentioned enough in our education, called angiotensin converting enzyme-2 (ACE2) exists near the surface of blood vessels and tissues of the lungs, brain, heart, and kidneys. Opposite of ACE1, ACE2 cleaves ATII into angiotensin (1-7). Angiotensin (1-7) is a vasodilator, has anti-inflammatory effects, and is an anti-thrombotic through its actions on the MAS-receptor. Think of this as the “good pathway,” reducing rates of thrombosis. But although ACE2 sounds like just the thing we need, unfortunately, ACE2 is also an entry point for coronaviruses, including SARS-CoV-2, which binds ACE2 and is then internalized into the cell.
As we all know by now, one tragic outcome of COVID-19 is thrombosis. One thought is that as more SARS-CoV-2 viruses bind ACE2, less ACE2 is available for AT1 to go down the “good pathway,” and simultaneously means that more AT1 gets converted to ATII- which we just learned causes thrombosis, oxidative stress, and inflammation.
As you may be aware, there is some current controversy on what to do with patients with COVID who are also on ACE-Is or ARBS. A deep dive into this is beyond the scope of this episode, but suffice it to say that most experts and guidelines advocate continuing ACE-I/ARB therapy in these patients (unless they are hypotensive, of course) due to the fact that these agents reduce the amount of ATII and its associated bad effects on thrombosis and other things. These agents also cause up-regulation of ACEII.
So, is more ACEII a good thing through its effects on reducing the amount of ATII and increasing the amounts of angiotensin (1-7), or is it a bad thing as it acts as an entry point of SARS-CoV-2 into cells? And if we like ACEII’s effects on reducing the amount of ATII, what are we doing actively infusing ATII into patients? Those are the questions that some experts have, and one day we may have the answer.
So at this time, there exists a plausible mechanism and a decent amount of data that ATII may be harmful for patients, especially in patients with COVID who are at a higher risk of thrombosis at baseline. However, we also have opposite data showing that ATII infusion works well in patients with COVID and septic shock- so the jury is still out on this one.
If that wasn’t enough, infectious complications are also a concern. Patients on ATII were more likely to have more than one infection, and more infectious events occurred overall in the ATII group vs the placebo group (60 events vs 35 events, P = 0.029). Also, those receiving ATII had higher rates of fungal infections (6% vs 1%, P = 0.035) per the package insert. Once again, the mechanism is unclear. But ACE1 is thought to increase resistance to infection, and giving ATII could lead to down-regulation of ACE1, making our patients more susceptible to infections.
There is also a concern that ATII can promote tachycardia and delirium, but rates of atrial fibrillation were the same between groups (13.5% vs 13.3%), and although delirium rates were higher in the ATII group (5.5% vs 0.6%), the overall rate of delirium was low at 3.1%. These two side effects are questionable, and we have to remember that all of the side effects discussed occurred in a short period of time. If you remember, the maximum duration of infusion was 7 days, with a large majority of patients getting the drug for < 3 days.
Overall, we’ve learned that ATII infusions will help your patients in septic shock by improving their BP/MAP and allowing you to wean off of background vasopressors a majority of the time. But remember, there was no effect on mortality rates, and ATII comes with a few concerning side effects that developed in a relatively short period of time. This can be especially devastating in the setting of COVID. But we have to keep in mind that at this time, the data is still inconclusive. And although most recommend continuing ACE-I/ARB therapy in patients with COVID, this obviously does not apply to hypotensive patients in septic shock. These patients need adequate organ perfusion, and as a matter of fact ATII in the setting of COVID has been shown to be beneficial in case reports and retrospective studies.
My overall view is that I’m convinced that ATII probably causes higher rates of adverse events, including thrombosis and infections. But I wouldn’t rule out its use as a third- or fourth-line vasopressor for critically ill patients running out of options, or in the setting of COVID quite yet. If you have a patient who remains hypotensive despite two or three vasopressors, ensure they are on adequate DVT prophylaxis and try ATII; it may be very beneficial for your patient. In the meantime, we will all await pending studies using recombinant ACEII infusions, AT1-receptor blockade, and ACE-I/ARB continuation or discontinuation in patients with COVID.
As always, thank you for your time. Please check out our website: errxpodcast.com. There, you can subscribe to our newsletter, which when we get enough subscribers, we will start sending out key points from previous episodes and other monthly tidbits.
