Episode 5- Subcutaneous neostigmine to treat Ogilvie’s Syndrome
Episode Summary:
In this episode, we look into the pharmacological treatment of Ogilvie’s Syndrome using subcutaneous neostigmine.
Show Notes:
Key Points:
“Subcutaneous neostigmine for the treatment of Ogilvie’s Syndrome“:
– Neostigmine given subcutaneously (SQ) is both safe and effective for the treatment of Ogilvie’s Syndrome
– The SQ route may be associated with less adverse drug events than the IV route and does not require ICU-level of monitoring for its administration; although it may take longer to see an effect compared to the IV route
– A reasonable protocol would to be to administer 0.5 mg SQ once, then repeat in 12 hours with 0.25 mg four times daily with a maximum of 3-5 doses prior to moving on to an alternative treatment strategy
References:
Neostigmine. In UpToDate. Waltham, MA.: UpToDate; 2020. www.uptodate.com. Accessed May 14, 2020.
Transcript:
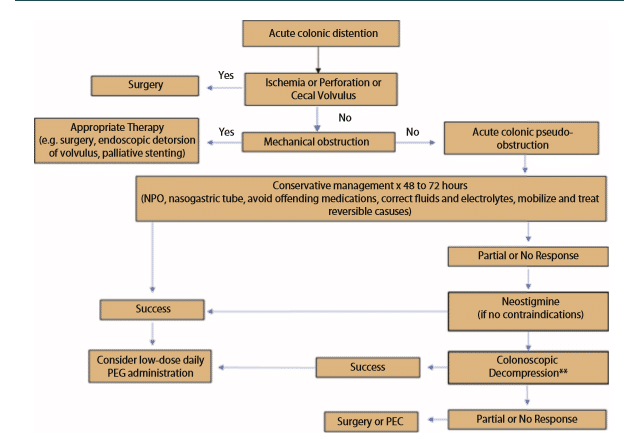
Hello everyone, and welcome to Episode 5 of ER-Rx. In today’s episode, we are going to discuss Ogilvie’s Syndrome, otherwise known as acute colonic pseudo-obstruction, as well as its treatment with subcutaneous neostigmine. First of all, what is Ogilvie’s Syndrome? Ogilvie’s Syndrome is defined as massive colonic dilation with signs and symptoms of colonic obstruction, but without overt mechanical blockage seen on imaging. Although it is still a very poorly understood entity, most researchers support the idea that there is an imbalance between sympathetic and parasympathetic innervation of the colon, likely due to parasympathetic suppression. Risk factors for developing Ogilvie’s include trauma, infections, cardiac disease, and underlying illnesses such as cancer. This is a very dangerous entity as well. Ogilvie’s can lead to intestinal perforation in 3% to 15% of cases, with a 50% mortality rate if this occurs.
For treatment, the goal is to decompress the colon to minimize the risk of perforation and ischemia. The first option we have for treatment is conservative management, which we will do usually for about two to three days. This includes NG (nasogastric) suction, giving the patient laxatives and enemas, hydration, a ‘nothing by mouth’ order, correction of electrolyte imbalances, and discontinuing contributing medications such as opioids, calcium channel blockers, and other medications with anticholinergic side effects. This can lead to a 77% to 96% success rate within the first two to three days. If conservative management fails, we do have the option of colonic decompression. This procedure has an 88% success rate. However, this comes with a 2% risk of perforation and a 1% risk of mortality. Therefore, at the moment, this procedure is used for patients who fail or who have contraindications to neostigmine; which brings us to my third treatment option, which is neostigmine. The question obviously leads to, “what is neostigmine?”
Neostigmine is an agent that inhibits the destruction of acetylcholine by acetylcholinesterases in the synapse. This leads to a direct cholinergic effect in the colon, where it promotes contractility and accelerates colon transit. Basically, neostigmine reverses the parasympathetic suppression that is thought to cause Ogilvie’s. Per the package insert, neostigmine can be given as a 2 mg IV push over three to five minutes. Neostigmine is contraindicated in patients that have peritonitis or mechanical obstruction, as well as those patients who have colon ischemia or perforation of the intestinal or urinary tract. There are also a number of relative contraindications including asthma, bradycardia, and beta-blocker therapy, and we’ll talk about why those are relative contraindications shortly. We know that IV neostigmine works in about 20 to 30 minutes and has up to a 94% success rate. However, giving neostigmine IV requires at least 30 minutes of cardiac monitoring in an ICU-level of care. This is due to the patient being at risk for developing bradycardia, AV block, hypotension, or even cardiac arrest. This need for heavy monitoring for IV neostigmine is a very important thing to remember, as it is the reason why there was interest in using alternative routes, namely the subcutaneous route, to administer neostgmine.
It is very well established that IV neostigmine is effective for treating Ogilvie’s based on randomized controlled trials, numerous meta-analyses, and both national and international guidelines. For example, the 2016 guidelines from the American Society of Colon and Rectal Surgeons list a strong recommendation that; “neostigmine is an appropriate next step for Ogilvie’s not responsive to conservative management.” However, the authors of this guideline do not mention subcutaneous administration of neostigmine. Nevertheless, we do have data from a number of studies that neostigmine given subcutaneously is both safe and effective. For example, in 2018, Kram, et al published a multicenter, retrospective, observational trial in 189 adult patients in three centers. The patients all had ileus, efractory constipation, or Ogilvie’s Syndrome. The authors found that the most commonly used regimen of subcutaneous neostigmine was 0.25 mg four times a day, with a wide range of both dosing and frequency. Their primary outcome, which was time to first bowel movement, was on average 29 hours, and about 1/2 of their patients needed five or more doses to reach this outcome. Interestingly, the authors found a dose-response relationship where the 0.5 mg dose seemed to be the most effective. This dose led to an average time to bowel movement of 12.5 hours, with 80% of their population having a bowel movement with the first dose. On the other hand, the 0.25 mg and the 1 mg dose both required more doses and had a longer time to first bowel movement. In terms of safety, none of the patients developed bronchospasm or cardiac arrest, although there was a 1% rate of bradycardia. In the two patients who developed bradycardia, one patient had a previous history of first-degree AV block already, and the second patient developed a first-degree AV block after the 14th dose of neostigmine. Even worse, that patient was on a beta blocker, which as I mentioned previously is a relative contraindication. To note, neither patient required any intervention.
The authors concluded that subcutaneous neostigmine is a reasonable alternative to the IV route. Although the time to first bowel movement was on average 29 hours versus the 20 to 30 minutes for the IV route reported in the literature, this is outweighed by the very low rates of cardiac side effects, namely bradycardia. The IV route comes with a bradycardia risk of 6% to 11%. My personal take on this study was that although only 10% of the cohort had a diagnosis of Ogilvie’s Syndrome (the other 90% had either ileus or refractory constipation), 80% of the total population was not in an ICU and were monitored by standard nursing interventions only. The two patients who develop bradycardia were at high risk of developing adverse drug effects anyway, and the second patient was given a lot of neostigmine at 14 doses. In conclusion, I would call for a 0.5 mg dose x1. Then if that doesn’t work, wait 12 hours and start 0.25 mg four times daily for two to five doses before moving on to the next treatment option. Citing this study, the 2020 guidelines from the American Society of GI Endoscopy discuss the use of neostigmine given both IV and subcutaneously for the treatment of Ogilvie’s Syndrome. As a final clinical pearl, in patients who respond to neostigmine, a small trial showed that polyethylene glycol (or Miralax), given once daily resulted in no recurrence of colonic dilation, whereas patients who received placebo had a 33% rate of recurrence. This practice of giving Miralax (or polyethylene glycol) is also supported in both of the guidelines that I mentioned previously, namely, the 2016 guidelines from the American Society of Colon and Rectal Surgeons, as well as the 2020 guidelines from the American Society of GI Endoscopy. And of course, for our international listeners, the 2015 guidelines from the Australasian College of Surgeons also recommends this practice.
In conclusion, subcutaneous neostigmine can be a safe and effective treatment for Ogilvie’s Syndrome, and we can avoid patient transfers to an ICU level of care just for monitoring. Giving it subcutaneously may take longer to work then the IV route, but the trade-off is a much lower rate of adverse drug events. All of these taken together will save the patient and the hospital time, money, and resources. The most effective dose seems to be 0.5 mg, followed by 0.25 mg four times daily. Personally, I would cap this at a total anywhere from three to five doses to reduce the risk of adverse drug events with higher and more frequent dosing. And in those patients who respond to treatment, starting them on daily Miralax can reduce the risk of recurrence. Finally, to keep our patients extra safe, mind the absolute and relative contraindications and be prepared to act if a patient develops any significant adverse drug events.
As always, thank you so much for your time. Please check out our website at errxpodcast.com where you can find a list of our references and read a full transcript of the episodes.
