Episode 26- “More Squeeze, Please:” Part 1: ATHOS-3 and me
Episode Summary:
In Part 1 of this “Mini Grand Rounds” series, we discuss Angiotensin II use for vasodilatory shock, reviewing the ATHOS-3 trial and real-world experiences.
Show Notes:
Key Points:
“‘More Squeeze, Please’: Part 1: ATHOS-3 and me”:
– We use two classes of vasopressors to treat vasodilatory/septic shock; catecholamines such as norepinephrine (NE)/epinephrine, and vasopressin. However, the renin-angiotensin-aldosterone system can be a potentially underutilized mechanism of raising blood pressures (BP) in these hypotensive patients
– The ATHOS-3 trial was an international, randomized, placebo-controlled trial using angiotensin II (ATII, Giapreza) vs placebo in addition to background vasopressors to improve BP/ mean arterial pressure (MAP) in patients in vasodilatory shock
– The study enrolled 321 patients; 163 received ATII and 158 received placebo. The primary endpoint (ability to achieve a MAP of >/= 75 mm Hg or an increase of the MAP by at least 10 mm Hg) was achieved in 70% of patients in the ATII group, but only 23% of the placebo group (P < 0.001)
– However, there was no difference in 7-day mortality (29% vs 35%, P= 0.22), 28-day mortality (46% vs 54%, P= 0.12), or SOFA scores between the groups
– My personal experience is that this agent does improve BP/ MAP, and it does so very rapidly. Usually ~ 50% of patients will be able to wean down or come off of some, if not all, background vasopressors. However, as in ATHOS-3, we have not seen an improvement in mortality rates
– With one vial of ATII costing ~$1,500, average total cost for drug alone was ~$6,000 at our site, with no patient using the agent for more than 5 days
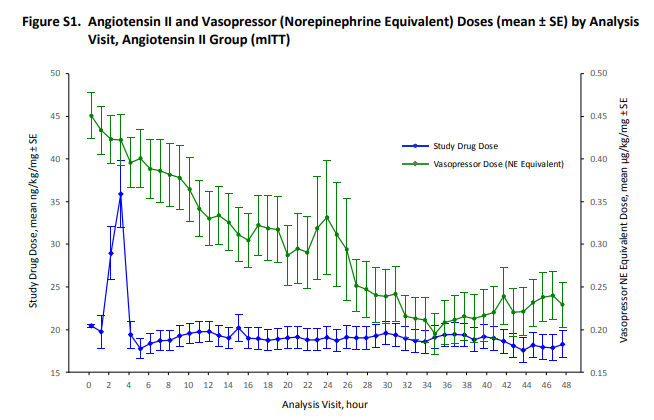
Both images above are from the Supplementary Appendix. The first image shows that over time, background vasopressors (green) were able to be weaned down, from an average of ~ 0.45 mcg/kg/min to ~ 0.25 mcg/kg/min.
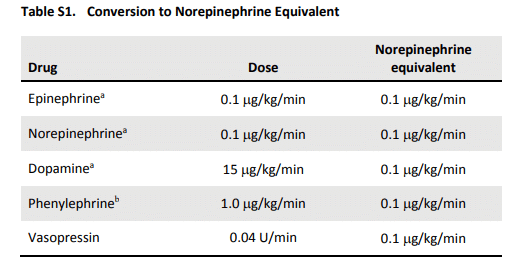
The second image is a table showing conversion to “norepinephrine equivalents,” which were discussed in the ATHOS-3 trial as well as this week’s episode.
Source: Angiotensin II for the treatment of vasodilatory shock. NEJM. 2017; 377: 419-430
References:
Transcript:
Hello and welcome to Episode 26 of ER-Rx. In part one of our “Angiotensin II Mini Grand Rounds” series, we discuss the use of angiotensin II (ATII; Giapreza) in the setting of vasodilatory shock. We’ll briefly discuss the “ATHOS-3” trial and its results, and then we’ll talk a little bit about what you should reasonably expect in your own hospital when and if you use this agent. In part 2 next week, we’ll discuss some important side effects of ATII and we’ll talk about what effects ATII can have in the setting of COVID.
Vasodilatory shock, including septic shock, is the most common type of shock, characterized by low blood pressures (BP) despite adequate cardiac output. Mainstays of treatment include administering broad spectrum antibiotics, adequate fluid resuscitation, vasopressor therapy, and glucocorticoids if needed, as well as a whole host of other recommendations as per the “Surviving Sepsis” guidelines. At this time, we use two classes of vasopressors; catecholamines such as norepinephrine (NE), epinephrine, and dopamine, and vasopressin. However, when we become hypotensive, our bodies activate another system, the renin-angiotensin-aldosterone system (RAAS)- a potentially underutilized mechanism of raising BP in our hypotensive patients. With this in mind, ATHOS-3 wanted to answer a simple question: “can the addition of ATII to background vasopressors improve BP?”
As a quick review, ATHOS-3 was an international, randomized, blinded, placebo-controlled trial in adult patients who had vasodilatory shock despite volume resuscitation who were also on high-dose vasopressors. “High-dose” vasopressors were defined as more than 0.2 mcg/kg/min of NE or equivalents for at least 6 hours. After checking baseline MAPs and APACHE scores, ATII or placebo solutions were started at a rate of 20 ng/kg/min and adjusted every 5 minutes during the first 3 hours to a MAP goal of 75 mm Hg. It’s kind of unclear exactly why a MAP of 75 was chosen, but it is likely so that they could detect a big enough difference between the groups. It was also a good way to assess the safety of higher-than normal doses. Either way, and more importantly, background vasopressors could not be increased during this time period unless absolutely necessary. Only after 3 hours could the background vasopressors be increased, along with adjustments to ATII; this time to a more normal MAP goal between 65-75. At hour 48, the study infusion was discontinued, and it was only allowed to be resumed if background vasopressors were increased by more than 0.1 mcg/kg/min of NE equivalents or if the patient became unstable. At that point, the medication could be resumed for up to 7 days, but note that only 13 patients received the agent for more than 72 hours.
In the end, the study enrolled 321 patients; 163 that received ATII and 158 who received placebo. The groups were well-matched at baseline, and both were severely ill with high APACHE II scores (median of 28) and high doses of baseline vasopressors (median of 0.3 mcg/kg/min NE equivalents). Over 95% of patients were on NE, and 70% were also on vasopressin. Unfortunately, the authors did not define the number of patients on “stress dose steroids” such as hydrocortisone.
The primary endpoint, which was the ability to achieve a MAP of >/= 75 or an increase of the MAP by at least 10 was achieved in 70% of patients in the ATII group, and only 23% of the placebo group (P < 0.001). At 3 hours, the ATII group had a higher MAP increase vs placebo (12.5 mm Hg vs 3 mm Hg; P <0.001). The authors found that if ATII worked, it worked very quickly, and the dose of ATII and/or background vasopressors was able to come down in 2/3rds of patients within 30 minutes.
Cardiovascular SOFA score improvement was greater in the ATII group (-1.75 vs -1.28, P=0.01), but other SOFA components did not improve significantly, and at 48 hours the total SOFA score increased in both groups (by about 1.05). The fact that only the cardiovascular SOFA score decreased is not surprising, as this score takes into account vasopressor doses, which we know were reduced in the ATII group. In multivariate analysis, the authors found that negative predictors to response to therapy were hypoalbuminemia, prior exposure to ARBs, and high background vasopressor doses; patients on < 0.5 mcg/kg/min of NE equivalents did better than patients on higher doses. However, there was no difference in 7-day mortality (29% vs 35%, P= 0.22) nor 28-day mortality (46% vs 54%, P= 0.12).
So, is this what we can expect in the real-world setting? The answer is; probably, hopefully? My hospital was lucky enough to be a study site for ATHOS-3, and after completion of the study we were able to continue to use ATII and it stayed on formulary. Our experience was similar to that of the overall ATHOS-3 experience. If ATII worked, it worked almost immediately. As the authors mention in their limitations, it was pretty easy for the team to figure out if the patient was getting an active medication or saline placebo most of the time. Remember that about a quarter of patients had increases in MAP at hour 3 due to placebo alone, and while that statement is true it still wasn’t that drastic of an increase, nor that fast of an effect when doses were adjusted up or down, so I still maintain that it was fairly easy to figure out which medication the patient was on.
We also found that ATII was used appropriately every time. Appropriate use was defined as the patient was thought to be adequately fluid-resuscitated and was also on 2 or 3 other vasopressors prior to ATII initiation. When it was used as a third-line agent, the other two pressors were always NE + vasopressin. When it was used as a fourth-line agent, patients were on a combination of NE plus vasopressin, plus either phenylephrine or epinephrine, which is consistent with guideline recommendations. We of course used MAP goals of 65 or BP goals of > 90 for most patients. We did find that less than half of the time, patients were able to reduce or discontinue background vasopressor dosing, not 2/3rds of the time as per ATHOS-3. We found similar mortality rates as in ATHOS-3, and no patient used the agent for more than 5 days. Using a cost per vial of $1,500, our average cost per patient for drug alone was ~ $6,000.
All in all, ATII use, both in studies and in real world situations, will probably increase your patients MAP or BP, and it will do so very quickly. So, the answer to the question of, “can ATII improve BP?” is a resounding “yes,” 70% of the time. This will come with the benefit of being able to wean off of background vasopressors, which is typically a great thing for our patients. However, don’t expect a change in SOFA scores or any mortality benefits at this time. Also, cost can be a concern, especially if you are using higher doses in bigger patients for longer periods of time.
As always, thank you for your time. Don’t forget to tune in next week when we discuss some important side effects of ATII, as well as the controversy of its use in the setting of COVID.
